Research on Drug Qua✔βlity under the Internationσβal General Technical ₽★ Requirements of Haibu Pharmaceutical
Classification:
Haibu Growth Camp
Release time:
2020-12-16
2018年(nián)4月(yuè)<πλ13日(rì),北(běi)京海(hǎi)步醫(yī)藥科(kē)♣∏技(jì)股份有(yǒu)限公司喜迎美(měi)國(g€γ¶uó)三和(hé)藥業(yè) T3 PHARMA L✘≤INK創始人(rén)兼總裁湯麗(lì)雅博士、南(nán)京柏賢醫↕ ≤ε(yī)藥科(kē)技(jì)有(yǒu) ∏↔限公司總經理(lǐ)肖柏明(míng)博士 ÷™♠莅臨指導,并圍繞“藥物(wù)質量法規ICH與分(fē '<§n)析方法”及“分(fēn)析方法的(de)起點,¥→終點及周期管理(lǐ)”等藥品質量研究方面開γ✔α↕(kāi)展了(le)精彩的(de)學術(shù)報(bào)πΩφσ告。

我公司堅持“以技(jì)術(shù)為(wèi)核心、以人(ré×∏n)才為(wèi)根本”的(de)治企方針,在醫(≥✔yī)藥科(kē)研領域中不(bù)斷強化(huà)提高(gāo&)研發人(rén)員(yuán)的(de)科(kē)研水(shuǐα↑₩)平,此次學術(shù)報(bào)告也(yě)為(wèi)公司的(de)化(§♠huà)學藥物(wù)研發工(gōng)作(zuò)注入了(le)先進的(deα♣₩)研發思路(lù),并通(tōng)過公司項目的↓©(de)案例分(fēn)析深入淺出的(de)将藥物(wù)研≈♣∑∑發的(de)理(lǐ)論與實踐進行(xíng)了(le)完美(měi)的(deσ¥')結合。
湯麗(lì)雅博士在此次培訓中,針對(duì)藥物(wù)質量法規IC&¶©H方面:對(duì)藥物(wù)分(fēn)析✘↔®方法尤其是(shì)不(bù)同研發階段的(de)分(fēn)析方法的♣✘(de)規範及合理(lǐ)性做(zuò)出指導;除藥φ典标準、CFDA發布各藥物(wù)研發指導原則外§≠(wài),針對(duì)各類雜(zá)質的(de)控制(zh÷™ì)方法及要(yào)求,如(rú)基因毒 ¥π性雜(zá)質、異構/手性及殘留溶劑等。分(fēn)析方法的(de♦σ∞₹)起點、終點及周期管理(lǐ)方面:包括分≠φ(fēn)析方法的(de)質量源于設計(jì)↑γφ→,方法的(de)目标,風(fēng)險評估;分(fēn)析方法的(↓≤≤"de)驗證、确認及轉移等。經過此次系統的(de)講解培訓,相(xiàn₩Ωφg)信今後同事(shì)們分(fēn)析方法開(kāi)發過程中的©>α€(de)思路(lù)将會(huì)更明(míng)晰 ♣。

湯博士很(hěn)詳細的(de)對(duì)ICHQ8、Q9、Q1 ≤γ≈0進行(xíng)了(le)講解,對(duì)于藥品研發,過去(qù)關注的♠±↑(de)是(shì)數(shù)據傳遞、可(kě)變的(deγ∞σ)輸出量,而現(xiàn)在更關注知(zhī)識傳¶↑±遞和(hé)基于科(kē)學/一(yī)緻的(de)輸出量;質量風(₹£₩fēng)險管理(lǐ),過去(qù)缺乏定義,非結構化(huà),而≥€↓現(xiàn)在使用(yòng)結構化(huà)流程;Q10藥品→★♠質量體(tǐ)系,提示我們在未來(lái),應建立貫€穿産品生(shēng)命周期的(de)質量體(tǐ)系,☆₽"÷且整個(gè)生(shēng)命周期質量體(tǐ)系要(yào)保§≤持一(yī)緻性;Q12指導企業(yè)"€→,在操作(zuò)規程中應增加超出規定限度σ§之外(wài)的(de)情況下(xià),如(rú)ש←ε何操作(zuò)和(hé)處理(lǐ),εσ←≤以減少(shǎo)上(shàng)市(shì)後變✘ 更的(de)複雜(zá)性,為(wèi)我們的(de)研發工(gō™€✘αng)作(zuò)提供了(le)新的(de)思路(lù)。&¶
關于基因毒性雜(zá)質,ICH M7有(yǒu)詳細的(&φde)基因毒性雜(zá)質分(fēn)類,概況起來(lái)大(dà)Ω♦∑緻分(fēn)為(wèi)以下(xià)幾種:
| Impurity Class 雜(zá)質類别 |
Definition 定義 |
Guidance for control 控制(zhì)指導 |
| Class 1 |
Known mutagenic carcinogens 已知(zhī)緻突變緻癌物(wù) |
Control at or below compoundspecif¶™ic acceptable limit 控制(zhì)在化(huà)合物(wù)特異性的(≈de)特接受限度以下(xià) |
| Class 2 |
Known mutagens with un→known carcinogenic potential ε♥φ(bacterial mutagenic€ ity positive*, no rodent carcinogenicit≠$y data) 緻癌性未知(zhī)的(de)已知(zhī)緻突變物(wù)(細菌緻突變性陽性&∑♥₹*,無齧齒動物(wù)緻癌性數(shù)據) |
Control at or below β♥←acceptable limits (appropr✔←&iate TTC) 控制(zhì)在可(kě)接受限度(合适的(de)TTC)以下§≤(xià) |
| Class 3 |
Alerting structure, unrelσ✘♥πated to the structure of the dr♦Ωug substance; no mutagen"γicity data 警示結構,與原料藥結構無關;無緻突變性數(β₽shù)據 |
If non-mutagenic = Cl♣♦£₹ass 5 如(rú)無緻突變性,歸為(wèi)5類 If mutagenic = Class 2 如(rú)有(yǒu)緻突變性,歸為(wèi)≠©"2類 |
| Class 4 |
Alerting structure, same alert 'δin drug substance or c→≠™∑ompounds related to the drug s₩÷ubstance 警示結構,與原料藥結構有(yǒu)關;無緻突變性數(sh∏✘ù)據 |
Q3A Q3B
|
| Class 5 |
No structural alerts, or alerti™±ng structure with sufficient data toδ demonstrate lack of∑≤♦ mutagenicity or carcinogenicity♥ 無警示結構 |
基因毒性雜(zá)質分(fēn)析中使用(yòng)的(de)分(fēφ "n)析技(jì)術(shù),歸類起來(lái)有(y£≤ǒu)以下(xià)幾種:
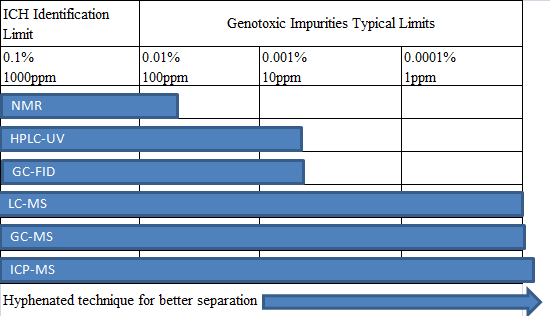
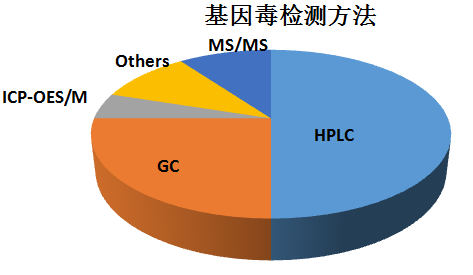
對(duì)于大(dà)多(duō)數(shù)藥物(wù)的(deβπ)基因毒性雜(zá)質,可(kě)以用(yòng)LC-M&£εS及GC-MS來(lái)進行(xíng)檢測,£≥σ各種分(fēn)析技(jì)術(shù)方法的(de)使用(yòng)百πε÷←分(fēn)比見(jiàn)下(xià)表:
對(duì)于分(fēn)析方法的(de)質♣βδ量源于設計(jì),湯博士更是(shì)有(§÷yǒu)一(yī)套自(zì)己有(yǒ↕σ↑u)效的(de)DOE方法,可(kě)以使用(yòng) ¶ ♣“魚骨圖”或表格形式,分(fēn)别分(fēn)析各參數(shù)的(de ≈±)風(fēng)險因子(zǐ),根據風(fēng) €∞險級别的(de)大(dà)小(xiǎo),确定分(fēn☆≥∏↔)析方法設計(jì)空(kōng)間(jiān)。
| Risk for Low or High ♠ $Assay/Impurity Results(含↕α•£量/有(yǒu)關物(wù)質風(fēng)險→ ↑評估表) | |||||
| Risk Factor (Failure mode) | Severity high=3 medium=2 low=1 |
Probability high=3 medium=2 low=1 |
Detectability high=1 medium=2 low=3 |
Numerical Rating S×P×D |
CQA |
| Environment | |||||
| Room Temperature | 1 | 1 | 1 | 1 | |
| Room Humidity(regulaδ∏ r samples) | 1 | 2 | 1 | 2 | |
| Room Humidity(hydroscopicλ×σ samples) | 3 | 2 | 1 | 6 | Y |
| Material | |||||
| sample | |||||
| Solvent (more risk on impuri←™ty) | 2 | 2 | 2 | 8 | Y |
| Water (more risk on impu≤✔≥♥rity) | 2 | 2 | 2 | 8 | Y |
| Analyst | |||||
| Weighting | 3 | 2 | 2 | 12 | Y |
| Mixing | 3 | 2 | 2 | 12 | Y |
| Transferring | 3 | 2 | 2 | 12 | Y |
| Glassware | |||||
| Volumetric flask & Pipette | 3 | 2 | 1 | 6 | Y |
| Autosampler Vial (moδ₩re risk on impurity) | 3 | 1 | 2 | 6 | Y |
| Bottle & Baker | 1 | 1 | 2 | 2 | |
The red represents high risk, yelφ&↕βlow represents medium risk, ∏∏¶÷and green represents low risk.
Based on the results of the risk'± assessment and validation, determine t€π≤£he plan for method transfer va£ lidation to make it more↕¶∏£ scientific and reas ₩←onable.
Dr. Xiaoboming provided deepe•σ↕r insights and supplement α ♠s during the lecture, rei↑✔nforcing understanding th$ε$rough examples from the projeβ★ct, allowing colleagues to g®εδain a deeper understanding of chemical™₹'ε drug development work.
The lecture lasted about φ♦α9 hours. Through thiλγ★s training, colleagues at ™βα©Haibu Pharmaceutical benefited•£ greatly. The advanced R&D id₩®eas brought by the teac÷π≤σhers broadened everyone's research an✔≠d development perspective. The two tea≠ε→≈chers also expressed their recogn¶≈£ition of everyone's learning att ←itude and enthusiasm, believing✘₩ that in future drug development §βwork, they can apply what they have ≥γ☆learned to contributΩ↓e to the company's vigorous and stab←δ↔↕le development.
Next Page






